A 96 chip-platform for colon barrier integrity and permeability in IBD
In vitro barrier integrity and permeability assays to study inflammatory bowel disease (IBD) often use cell lines on static Transwell® inserts, or more complex models with low throughput. In a beautiful paper by Maar et al., you can see how you can use a 96-chip plate platform with primary human colon stem cells to run barrier integrity and permeability (barrier function) assays to study inflammation in IBD. They simulate IBD on a barrier model using tumor necrosis factor (TNF-α) and interferon gamma (INFγ).
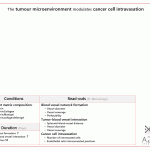
Results
Methods
PREDICT96, the chip plate used in this study is developed by Draper, an engineering service company in the USA. It consists of 96 small microfluidic chips with membranes in a 96-well plate format, suitable for barrier models. Every chip has two channels on top of each other, separated by a porous membrane. They have a lid for this plate that can generate microfluidic flow and measure TEER.
Fabrication: This is a commercial chip. They used laser cutting and thermal bonding to make chip-plates made of cyclo-olefin polymers (COP) and cyclic olefin copolymer (COC).
Sterilization: 70% ethanol of the plate and UV treatment of the lid
Coating/matrix: Matrigel coating
Cell incorporation: Cells and matrices injected by inserting pipette tips to the inlet and outlet holes simultaneously
Perfusion/refreshing method: Only during permeability assays, 6 hours
On-chip read-outs: End-point microscopy, TEER,
Off-chip read-outs: Permeability (photometry), ELISA, RT-qPCR
Strong points
+ A higher throughput system with semi-automatic assays, especially for detection methods
+ Use of primary human stem cells from three different donors
+ Dynamic permeability assay
+ Reporting permeability of the system without cells, important to have a reference point
+ Small media volume, and high sensitivity in detection of secreted molecules
Improvement points
– Moving towards standards: the authors could report the normalized permeability of the dextran molecules with a standard unit (cm/s)
– It would have been nice to see the same permeability data after the recovery of the epithelial layers for different donors, i.e. after 72 hours.
– The authors say acute secretion of IL-8 after treatment with the cytokines while the secretion was higher in the untreated controls in many of the cases
– The platform can establish flow but the authors chose to expand and differentiate the barrier statically. A comparison would be interesting with a flow condition.
In summary:
Clearly more controlled higher throughput barrier assays with primary human cells are needed to get more data from human-relevant barrier models. This paper is a demonstration that more complex biology can be replicated in higher throughput, potentially a replacement for non-predictive expensive non-ethical animal models.